Cultivation of Phenion® FT Skin Models
Researchers from Henkel, BASF, the German Federal Institute for Risk Assessment (BfR) and Procter & Gamble [1] have adapted the comet assay [2] methodology (a test allowing to detect genotoxic hazard) to two reconstructed full thickness human skin models: the EpiDerm™- and Phenion® Full-Thickness Skin Models. The resulting “3D Skin Comet assay” has successfully completed the first phase of a comprehensive evaluation process. Results of this initial study show that full-thickness skin models can be used to evaluate possible DNA damage (or ‘genotoxicity’) induced by new raw materials with a high degree of predictability and reproducibility.
Eight chemicals were first tested in several laboratories each using Henkel’s Phenion® Full-Thickness Skin Model, informing several validation modules. Ultimately, the 3D Skin Comet assay demonstrated a high predictive capacity and good intra- and inter-laboratory reproducibility with four laboratories reaching a 100% predictivity and the fifth yielding 70%.
The aim of the research was to gather enough data showing that the 3D Skin Comet assay can be used as a new in vitro tool for following up on positive findings from the standard in vitro genotoxicity test battery for dermally applied chemicals, ultimately helping to drive the regulatory acceptance of the assay. To expand the database, the validation will continue by testing an additional 22 chemicals.
Reproduction of human skin
According to Henkel, which developed the Phenion® Full-Thickness Skin Model, bioartificial skin models are ideally suited to serve as reliable test system for a broad spectrum of applications as they are based on human skin cells and are organised in a three-dimensional environment, which well reflects human native skin. The Phenion® Full-Thickness Skin Model consists of both a fully differentiated epidermis, the outermost layer of the skin, and the underlying dermis that contains a natural, collagen-based connective tissue. The model therefore resembles human native skin in a variety of anatomical and physiological properties.
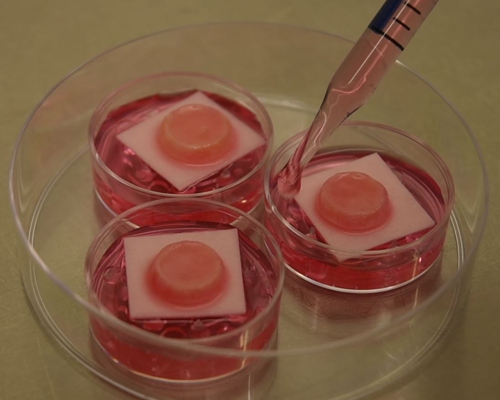
Seperate cultivation of Phenion® FT Skin Models during chemical exposure
“Inventing innovative products requires reliable test methods. Therefore we, at Henkel, are engaged in developing new testing methods that allow us to assess the safety of ingredients,” said Dr. Dirk Petersohn, Director of Biological & Clinical Research at Henkel Beauty Care. “The excellent study results are an important step towards the acceptance of the 3D Skin Comet assay as an officially approved test method,” he concluded.